QUALITY CONTROL
Quality control is the process of ensuring the consistency, safety, and efficacy of biopharmaceutical products through rigorous testing and validation. Allianz Bio provides high-quality reagents and analytical tools to support research and development in biopharmaceutical quality control.

Columns & Standards
Allianz Bio provides chromatography columns and reference standards essential for quality control, enabling accurate separation, identification, and validation of biopharmaceutical components to ensure regulatory compliance.
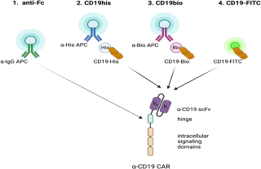
Characterization of CAR
For the characterization of CAR T-cells, Allianz Bio provide comprehensive solutions for evaluating critical quality attributes such as CAR expression, transgene copy numbers, and T-cell functionality.

Titration Kits
Our AAV2 titration kits use qPCR or ELISA to accurately measure viral concentration, ensuring the consistency and safety of gene therapy products.
Biomarker Kits
Allianz Bio offers biomarker kits that enable precise detection and quantification of disease-related indicators, supporting monitoring of disease progression, and evaluation of therapeutic efficacy.
Biosimilars Elisa Kits
Allianz Bio’s Biosimilars Elisa Kits enable accurate monitoring of therapeutic drug levels and anti-drug antibodies.
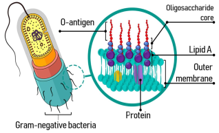
Endotoxin Detection
Our Endotoxin detection kits are used to identify and quantify bacterial endotoxins in pharmaceutical and biological products.
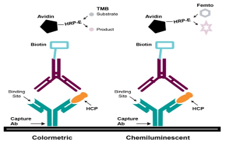
HCP/HCD
Our HCP and HCD kits are designed to detect and quantify host cell proteins and host cell DNA in biologics, ensuring product purity and regulatory compliance.
Residual Detection Kits
Allianz Bio offers Residual Detection Kits designed to identify trace impurities in biopharmaceutical manufacturing, ensuring product safety and regulatory compliance across antibody, CGT, and vaccine production.
.jpg)